Dupixent Now Approved for Chronic Spontaneous Urticaria
As of April 2025, the Food & Drug Administration (FDA) has approved a medication called Dupixent for the treatment of chronic spontaneous urticaria. This biologic drug, generically known as dupilumab, has been used until now to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, and a few other conditions. Find out more about Dupixent and how it might help you below.
What Is Dupixent and How Does It Work?
Dupixent is a human monoclonal antibody, so it’s made of living cells. It prevents the signaling of crucial drivers that trigger inflammation. It’s administered via injections and can be taken alongside other medications. For example, people who have asthma can take Dupixent at the same time as inhaled corticosteroids.
Usually, treatment begins with two injections. This loading dose is the largest amount you’ll likely take before tapering the drug.
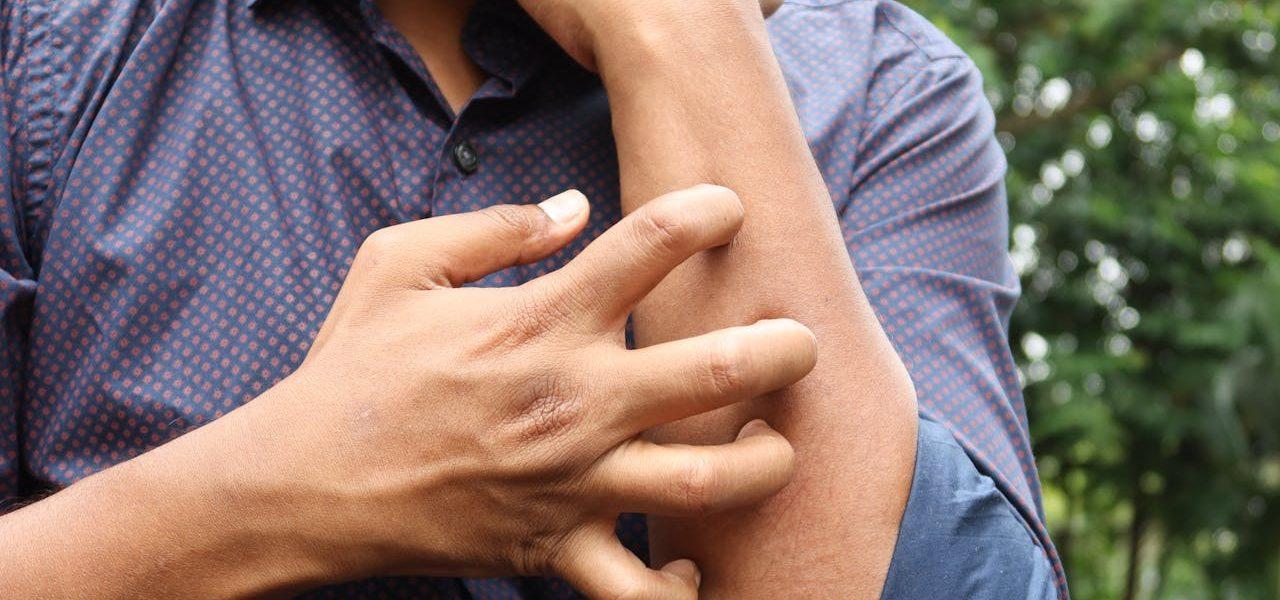
What Is Chronic Spontaneous Urticaria (CSU)?
Chronic spontaneous urticaria (CSU) is a skin condition that causes repeated itchy and raised hives or deep swelling without any apparent cause or trigger. The hives can appear anywhere on the body, and they can be uncomfortably itchy. If you get deep swelling, it tends to appear on your face, feet, neck, and hands.
To receive a CSU diagnosis, you must experience symptoms daily or almost daily for no less than six weeks. The symptoms tend to disappear in about 24 hours, but they reappear in other parts of the body. The hives don’t leave any kind of scarring, but they can make people self-conscious while also causing discomfort.
Many people who struggle with CSU have difficulties sleeping because of the itching. School or job performance can be impacted. Without treatment, quality of life can suffer.
Why Does CSU Happen?
CSU occurs because of an immune response. Mast cells in the blood release histamine into the skin tissue, leading to a red and itchy rash. People with autoimmune conditions tend to make up a large number of those who have CSU. Stress, infections, and certain medications may increase your risk of developing this condition.
What Does the New FDA Approval Mean for Patients?
The FDA has approved the use of Dupixent for people 12 years old and older who have CSU and who remain symptomatic despite receiving histamine-1 (H1) antihistamine treatment, the standard treatment option for this condition.
How Did the FDA Come to This Conclusion?
The approval comes after the FDA assessed data from two Phase 3 clinical trials involving people who struggled to manage CSU despite receiving antihistamine treatment. In the trials, Dupixent was added to the traditional treatment. The combination of the biologic and antihistaminic medications provided better results than the latter alone.
The studies showed that at 24 weeks, this combined treatment increased the likelihood of a patient having a controlled condition compared with participants who received a placebo.
Dupixent is also considered to be safe, with only a few potential adverse effects. It has received approval from 60 countries for the treatment of CSU, as well as many other conditions.
Why Is Cost Still a Major Barrier for Dupixent?
Although Dupixent is now available for doctors to prescribe to patients with chronic spontaneous urticaria, it is an expensive biologic medication that has an elaborate manufacturing process. List prices can be as high as $4,000 per carton.
For patients, this likely means struggling to afford the injections they need to get relief from CSU symptoms. People on Medicare or Medicaid plans can have an uphill battle trying to obtain prior authorization, which is a requirement for this medication, and it’s virtually impossible to afford it out-of-pocket.
Will the Price of Dupixent Go Down Over Time?
Dupixent prices are unlikely to decrease anytime soon because there isn’t enough competition. Few medications can target all of the conditions that Dupixent does, and the ones that do are not as widely available.
Find Affordable Relief From CSU
If you have CSU and are finding it difficult to afford Dupixent injections when you need them, the team at Simplefill can help. We offer patient assistance programs that can aid you in finding ways to afford your medications. For a broader look at how regulatory decisions affect drug prices, see our blog: New FDA Rules Could Raise Medication Prices & Slow Access.
Get Affordable Access to Prescription Medications
Simplefill is a full-service prescription assistance company that researches, qualifies, and maintains patients’ enrollment in all sources of assistance available to them.
Apply today by calling 877-386-0206. A caring Simplefill representative will contact you within 24 hours to discuss your application and, if qualified, enroll you in the program.
Frequently Asked Questions
How does Dupixent help treat CSU?
Dupixent blocks specific immune signals that trigger inflammation and hives. In clinical trials, adding Dupixent to standard antihistamines led to better symptom control than antihistamines alone.
How much does Dupixent cost without insurance?
Dupixent can cost up to $4,000 per carton before insurance or assistance. Coverage often requires prior authorization, and many patients struggle to afford it without help.
Is Dupixent safe for CSU patients?
Yes, clinical trials show Dupixent is generally safe, with a low rate of adverse effects. It’s FDA-approved for patients 12 and older who remain symptomatic despite antihistamines.
What can I do if I can’t afford Dupixent?
You can apply to patient assistance programs. Organizations like Simplefill help manage the application process and keep your enrollment active so you don’t lose access to treatment.
blog